How an RFID Antenna Works: It’s Your System’s Translator
33Discover how an RFID antenna works as the critical translator in your system. Learn about signal transmission, power harvesting, and data collection in simple terms.
MoreAll RFID Product
In clinical practice, accurately confirming whether patients are taking medication as prescribed has always been a challenge. Significant discrepancies exist in patient reports, medication box records, and medication recall during follow-up visits. For patients requiring long-term, regular medication, this uncertainty often directly impacts treatment outcomes.
Recently, a research team at MIT proposed a new technological approach, combining passive RFID technology with biodegradable materials to design an RFID capsule that can be swallowed with medication to record whether the drug has actually entered the stomach and begun to dissolve. This system is named SAFARI.
It is only identified “after swallowing”.
The SAFARI capsule has a simple structure. Inside the capsule is a passive RFID tag, coated with a radio frequency shielding layer composed of cellulose and metal microparticles. Before the capsule enters the stomach, this coating blocks the radio frequency signal, making the RFID tag unreadable from the outside.
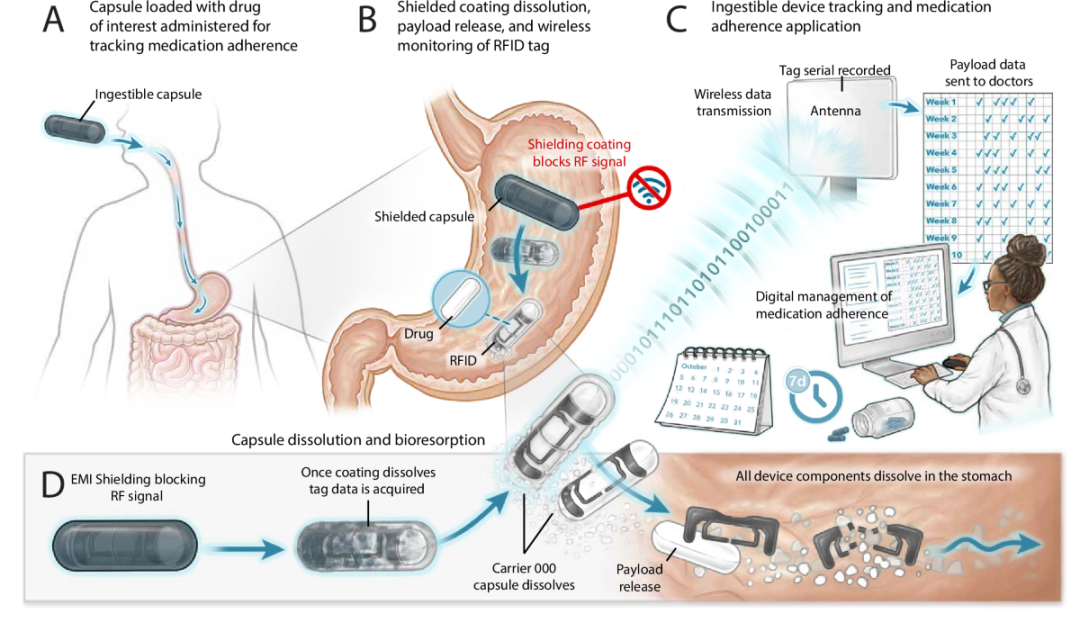
Figure 1: Schematic diagram of a capsule-based biodegradable medication adherence tracking system and its envisioned clinical application scenarios.
When the capsule reaches the stomach, the shielding layer gradually dissolves under the action of gastric juices, releasing the radio frequency signal, which an external rfid reader can then identify. This design avoids recording before swallowing, accidental contact, or environmental interference, ensuring a mechanistic correspondence between “identified” and “medication taken.”
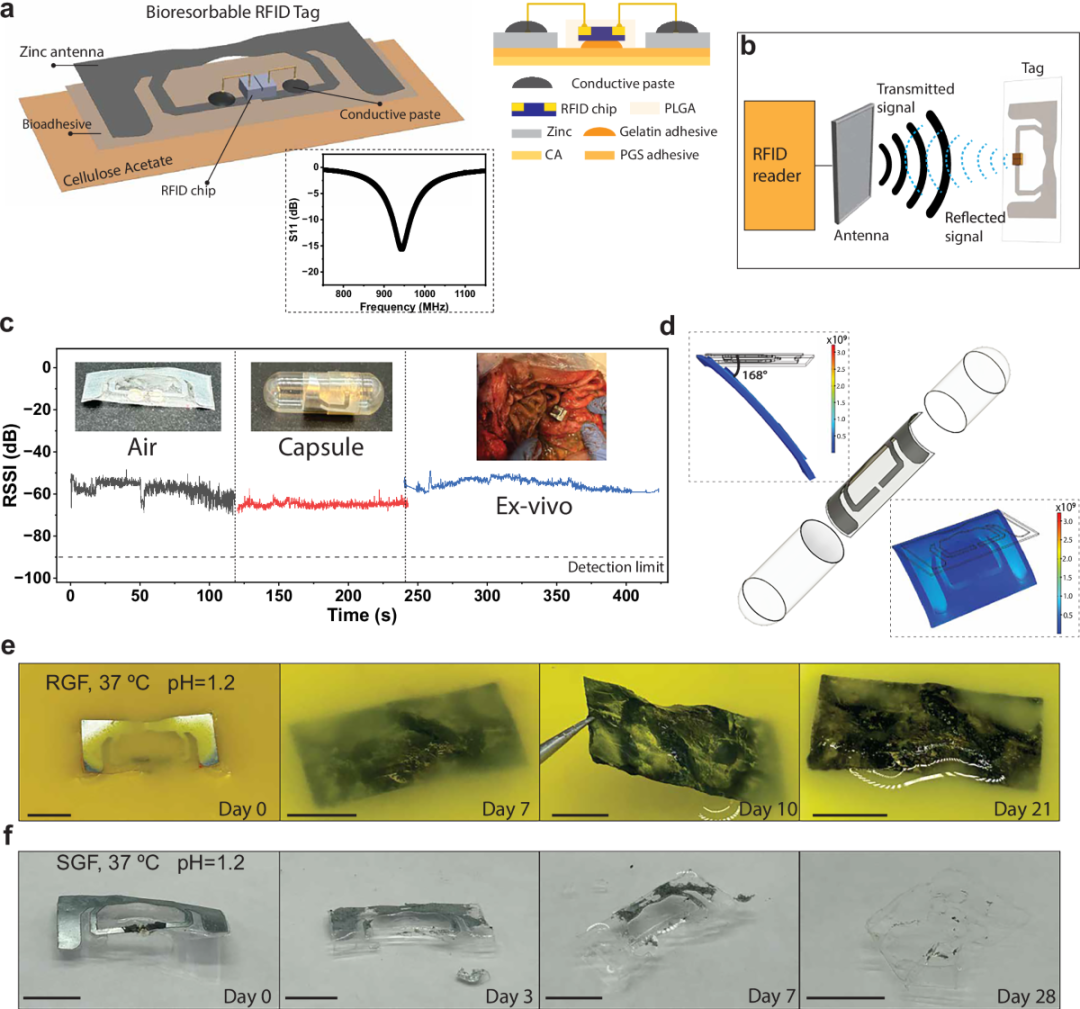
Figure 2: Electrical characteristics of a bioabsorbable RFID tag.
Minimizing Material Residue in the Body
Most previous ingestible electronic devices used non-degradable materials, which were completely excreted from the body. This is not a problem for short-term studies, but in scenarios requiring long-term monitoring, repeated ingestion of non-degradable electronic components may raise safety and environmental concerns.
SAFARI prioritizes absorbable or biodegradable materials. The antenna is made of zinc foil, the shielding layer is based on a cellulose system, and the capsule body uses conventional gelatin or HPMC. In in vivo experiments on a pig model, the capsule and metal structure gradually decomposed in the stomach, releasing zinc and molybdenum levels lower than those from daily dietary intake, with no abnormal changes observed in blood parameters. Ultimately, only the extremely small RFID chip remained, excreted through the digestive tract.
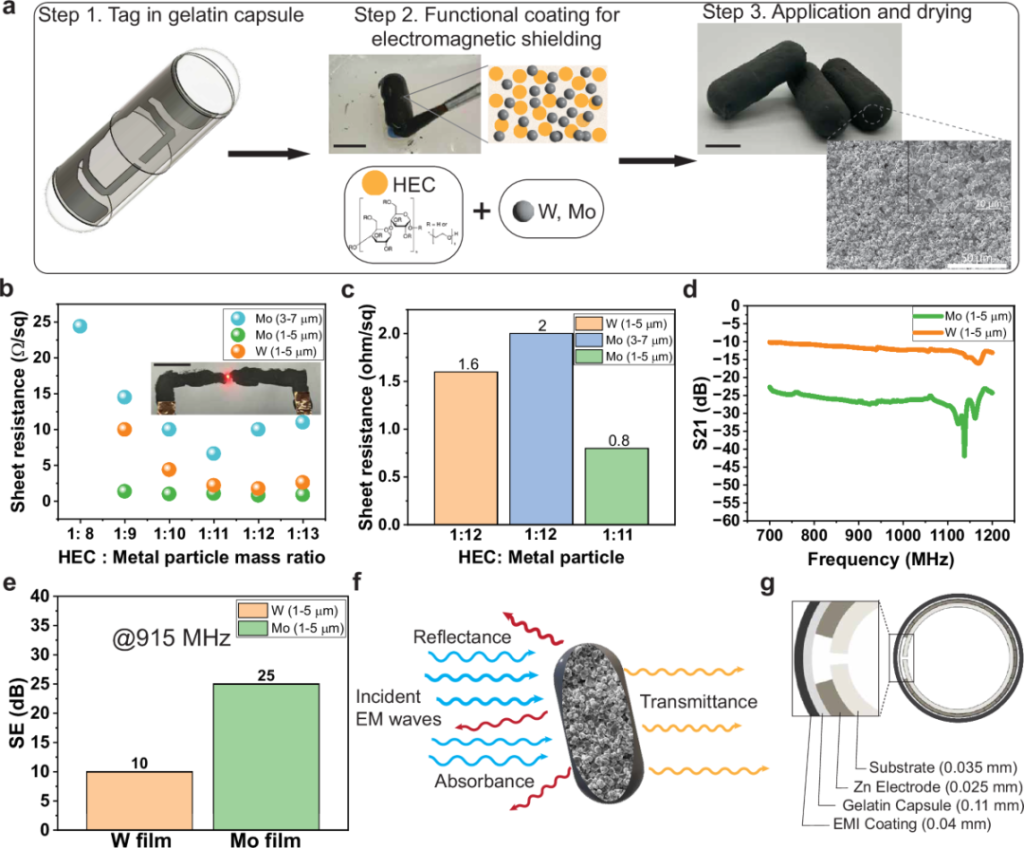
Figure 3: Preparation and electrical performance characterization of the cellulose-based electrical shielding material.
Tools for Specific Clinical Scenarios
The research team did not position these capsules as widely used consumer products. Instead, they limited their applications to treatments requiring extremely high adherence, such as treatment of infectious diseases like tuberculosis and HIV, immunosuppressive medication after organ transplantation, and prescription drug management sensitive to abuse risks.
In these cases, confirming “whether the medication was actually taken” is itself important clinical information, rather than simply a behavioral record.
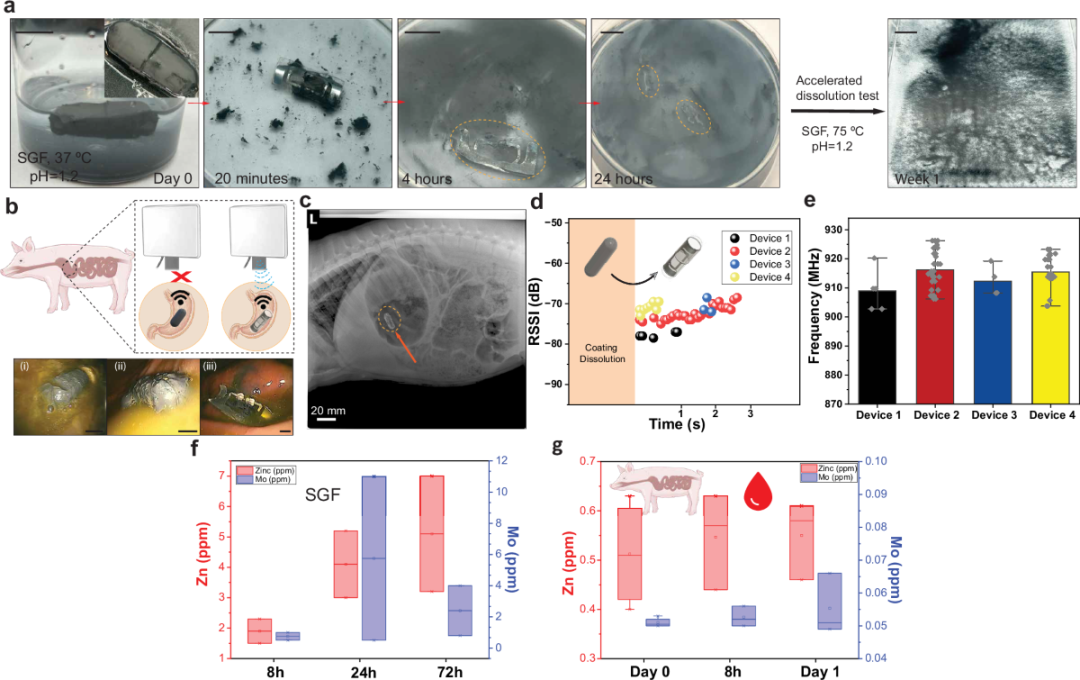
Figure 4: In vivo demonstration of the bioabsorbable cellulose-based RFID tag.
A Direction Gradually Moving Towards Practical Applications
From a technical perspective, SAFARI did not introduce complex active circuitry or battery systems, but instead utilized passive RFID and the material dissolution process to form a simple and clear triggering logic. This approach is closer to real-world conditions in terms of manufacturing, size control, and compatibility with existing pharmaceutical processes.
While this study doesn’t provide a definitive answer, it demonstrates a line of thought: in swallowable electronic devices, functionality doesn’t need to be complex; the key is long-term, safe use, addressing only the truly necessary issues.
In the long-standing area of medication adherence, where reliable tools have always been lacking, this attempt deserves continued attention.
How to Integrate Medication Behavior into Management Processes
In practical applications, recording whether medication was actually taken often requires integration with a front-end medication management system to form a complete information chain. For example, in hospitals or research settings, medications are typically managed centrally using RFID smart medicine cabinets, recording the time, personnel, and batch of each retrieval. Cykeo provides RFID smart medicine cabinet solutions for routine medical management, enabling the information recording of the medication retrieval process.

From the moment the medication is removed from the RFID medicine cabinet to the swallowable RFID capsule being identified in the stomach, the data from both ends can form a corresponding relationship in the system: when the medication was retrieved and whether it was taken within the expected timeframe are clearly recorded. This approach does not change the clinical process; it simply transforms the previously ambiguous medication use behavior into traceable information.
Discover how an RFID antenna works as the critical translator in your system. Learn about signal transmission, power harvesting, and data collection in simple terms.
MoreLearn how to integrate handheld RFID readers with ERP systems like SAP or Oracle. Follow expert steps for data sync, API setup, and real-time inventory updates.
MoreDiscover the top 10 advantages of UHF RFID readers for supply chains in 2024. Boost efficiency, accuracy, and ROI with Cykeo’s cutting-edge RFID solutions.
MoreGet a clear answer to how far can RFID be read. We break down the real ranges for LF, HF, and UHF systems and what factors actually affect reading distance.
More